Jennifer Ann Yang, MPP, CHC, CPHQ (jennifer.yang@state.mn.us) is the Deputy Chief Compliance Officer at the Minnesota Department of Human Services in St. Paul, MN.
Serving as the compliance officer for a psychiatric hospital, which was under a Systems Improvement Agreement (SIA) with the Centers for Medicare and Medicaid Services (CMS) in the past two years, I had the opportunity to develop and implement a survey readiness framework to assist the facility in preparing for two unannounced, full Medicare certification surveys. With extensive pressure and a tight timeline of approximately six months, the survey readiness team went through a journey of major obstacles and challenges, but nevertheless succeeded.
In this article, I will share the survey readiness framework and provide practical tools and examples. With this framework, the facility successfully completed the SIA requirement of passing both surveys without any condition-level findings. Although this framework focuses on CMS Conditions of Participation (CoPs), other regulatory/accreditation standards can be applied to the framework.
Survey readiness is based on two parts: survey preparation (the year-round efforts prior to survey) and survey performance (the facility’s use of resources and response during the survey). Survey preparation depends largely on strong organizational skills and using practical project tools to move teams toward being in compliance and maintaining compliance. Leadership must build a culture that fully accepts and operates on being prepared for a survey 365 days of the year. Staff performance is critical during the week of survey. During this week, the facility must use resources and skills in the most optimal manner. The facility’s survey results will depend largely on how effectively and quickly staff respond to surveyors’ requests, and how knowledgeable and skilled staff are in navigating surveyors through patient charts and answering questions and concerns.
Survey preparation
The survey readiness framework has five phases (i.e., assessment, planning, execution, demonstrated competency and validation checks, and taking action) within an ideal timeline of 12 months — a continuous, year-round effort.
Assessment (2 months)
A facility-wide assessment should be conducted based on a comprehensive review of the regulatory standards. It is recommended to conduct a facility-wide assessment at least once every year. Assessments can be based on a comprehensive review of standards, mock surveys, chart reviews, patient observations, or a combination thereof. This effort includes:
-
Establishing 12 teams, each team focusing on one or two standards;
-
Conducting small workgroup meetings for each team to review the standard(s) and for the facilitator to ask mock survey questions (directly pulled from the CMS Manual) about how the facility complies with the standards;
-
Identifying the corresponding policies, procedures, and processes for each standard; and
-
Reviewing and analyzing the CMS survey protocols to help identify gaps and opportunities for improvement.
Key tools used in this phase include:
-
CMS State Operations Manual (Appendix A and Appendix AA), including survey protocols, guidelines, and interview questions.
-
CMS worksheets: discharge planning, quality assessment and performance improvement, and infection control.
A critical deliverable for this phase is to develop 12 teams with each team owning a chapter binder — a collection of policies, procedures, and pertinent documents related to the CMS standard(s). For example, a team of six (with the social work director as the lead) is responsible for reviewing, understanding, and ensuring the facility’s compliance with the patient’s rights standard (42 CFR § 482.13).
Planning (1 month)
Based on the results of the assessment, leadership and the compliance officer strategize survey readiness efforts in preparation for the upcoming surveys. This phase includes:
-
Developing a survey readiness plan that reflects both long-term goals and high-priority goals that require immediate action;
-
Developing a survey readiness tracking tool to help organize and monitor all identified concerns and corrective actions based on the results of assessment;
-
Identifying chapter team leads to establish accountability for compliance-related improvements and corrections; and
-
Establishing a survey readiness committee at a leadership level (includes all chapter leads) to ensure oversight of corrective actions and continuous compliance.
Execution (4 months)
The execution phase is initiated with the convening of the survey readiness committee. This committee reviews the survey readiness tracker (SRT) on a regular basis — monthly is recommended. The hospital/facility administrator or a member of leadership is the chair of the survey readiness committee. The compliance/quality staff are directly involved in the committee to help coordinate meetings, facilitate discussions, and to assist the team with achieving project milestones and critical compliance-related deliverables. This committee reviews and discusses action items across all 12 chapters to ensure coordination and collaboration when applicable.
During this phase, the small workgroup meetings continue to take place on a bi-weekly or periodic basis. The role of the compliance officer is to ensure small workgroup meetings are scheduled and work is accomplished within deadlines. The 12 small workgroups will continue to function with a hands-on approach to implement and monitor compliance activities. Each workgroup is responsible for maintaining their chapter binder — policies, procedures, processes, data, committee meeting minutes, and other documentation related to their regulatory standard(s). This workgroup identifies missing, dated, and duplicative policies that are inaccurate and/or inconsistent. The workgroup helps draft and revise policies for leadership approval, and then provides appropriate staff education and training upon full approval. The small workgroups (via their chapter leads or the compliance officer) will share information and bring concerns to the survey readiness committee. Likewise, the survey readiness committee will circle back to the small workgroups about decisions made and further actions needed.
The compliance officer, in coordination with the chapter leads, is key in this communication process.
Additionally, the compliance officer’s role in this phase is to manage the SRT by making all updates and noting all progress. The SRT (see Table A) is a robust tool with clearly identified risks, concerns, and opportunities for improvement; specific actions to eliminate risks/concerns or make improvements; clearly established deadlines; and the corresponding status of each item (percent complete). This tool will help facilitate the meetings by focusing on high priority items first, and then all other past due items.
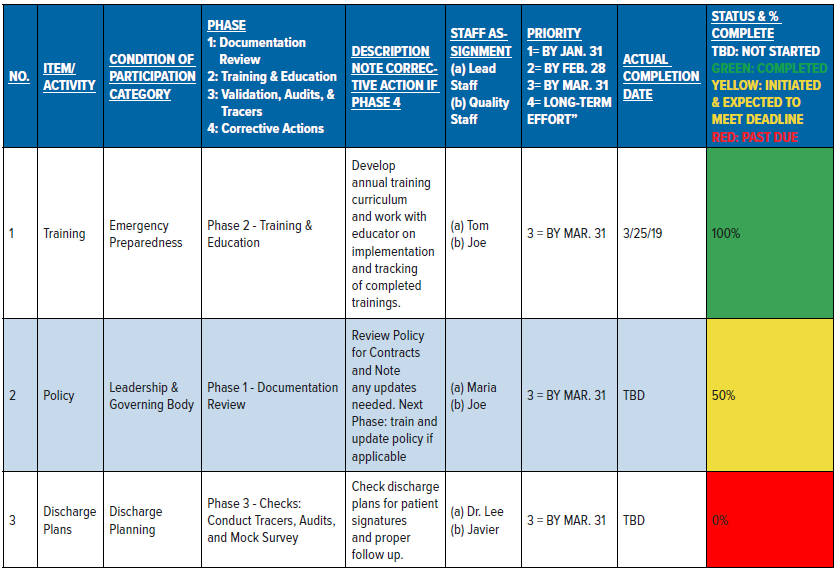
Demonstrated competency and validation checks (3 months)
Upon updating policies, procedures, and practices and receiving necessary trainings, staff are expected to demonstrate competency and knowledge.
The Quality and Compliance staff conduct tracers, audits, and checks on staff competencies/knowledge to validate that actions are completed, and to ensure continuous compliance and sustained improvements. Moreover, Quality staff collect and analyze audit data to determine trends and identify other opportunities for improvement. Data should inform leadership about progress and achievements, and also drive decision-making for effective interventions and additional actions. Key tools that are useful in this phase include tracer tools, audit tools, and competency checklists.
In this phase, the compliance officer validates that each binder has updated/accurate policies, procedures, and pertinent documents organized by applicable regulatory standard. How chapters are organized will depend on the size, structure, operations, and type of the facility. As an example, under the CMS standards for hospital and psychiatric hospital, the 12 chapters can be organized in this manner:
-
Emergency Management: 42 CFR § 482.15
-
Food and Dietary: 42 CFR § 482.28
-
Infection Control: 42 CFR § 482.42
-
Governing Body & Leadership: 42 CFR § 482.11, 42 CFR § 482.12, 42 CFR § 482.26, and 42 CFR § 482.27
-
Medical Records, Discharge Planning, & Rehabilitation Services: 42 CFR § 482.24, 42 CFR § 482.43, 42 CFR § 482.56, and 42 CFR § 482.61-62
-
Medical Staff: 42 CFR § 482.22 and 42 CFR § 482.62
-
Nursing Services & Organ, Tissue, and Eye Procurement: 42 CFR § 482.23, 42 CFR § 482.45, and 42 CFR § 482.62
-
Patient’s Rights: 42 CFR § 482.13
-
Pharmacy Services: 42 CFR § 482.25
-
Physical Environment: 42 CFR § 482.41
-
Quality Assurance and Performance Improvement Program: 42 CFR § 482.21
-
Utilization Review: 42 CFR § 482.30
Taking action (2 months)
Based on the results of the checks and audits, items are discussed at the next survey readiness committee meeting. Findings from the tracers, checks, and audits are incorporated into the SRT to ensure further actions are implemented and monitored. Oftentimes, performance improvement projects are initiated for more complex corrective actions and long-term efforts. The SRT is a tool that should be used on a continuous, ongoing basis and serve as a roadmap for the facility’s compliance efforts. The chapter binders should be maintained year-round, with the identified workgroup and team lead responsible for updating policies and other items as necessary.
