Sarah M. Couture (sarah.couture@ankura.com) is Managing Director and Brian D. Annulis (brian.annulis@ankura.com) is Senior Managing Director at Ankura Consulting Group in Chicago.
Since the inception of ClinicalTrials.gov, “a database of privately and publicly funded clinical studies conducted around the world,” in 2000,[1] the volume of registered clinical trials in the United States has skyrocketed. As of August 9, 2020, there were 115,883 active clinical trials in the United States.[2] Commensurate with the growth in registered clinical trials, there has been increased oversight and regulatory focus on clinical trials, including Common Rule updates,[3] billing compliance enforcement, scientific misconduct investigations, conflict of interest inquiries, and kickbacks and related enforcement actions, to name a few. As institutions pursue clinical research opportunities, whether industry sponsored or investigator initiated, it has never been more important for institutions to have solid administrative and operations processes to support their clinical research billing—what the authors of this article call research operations core (ROC) workstreams. Well-grounded processes not only help mitigate billing compliance risks, but also support efficient clinical research and contribute to the overall research strategy and financial success of the institution.
Our country is replete with world-class clinical research programs and brilliant scientists pursuing novel clinical discoveries and innovative treatments such as new drugs and medical devices. Behind the names and even potential fame and glory of these clinical research programs and investigators are the necessary administrative functions and staff that support them. While these ROC workstreams and staff may be far less flashy or recognized than the science or the scientists, they are the essential backbone of successful clinical research programs.
Clinical research billing and revenue cycle support operations
In many ways, the overall success of a clinical research enterprise rises and falls on the strength of its administrative support functions, including the research revenue cycle. While research leadership support, investigator commitment and passion, staff engagement, and patient participation are all essential, a clinical research program cannot realize its full potential—or ensure its regulatory compliance—without intentional focus; collaboration across silos; and active pursuit of excellence in clinical research operations, administration, and the revenue cycle. This article discusses some common pitfalls across the various ROC workstreams and identifies best practices to ensure billing compliance and to strengthen the efficiency and value of research operations. We organize those workstreams into pre-trial and post-study categories.
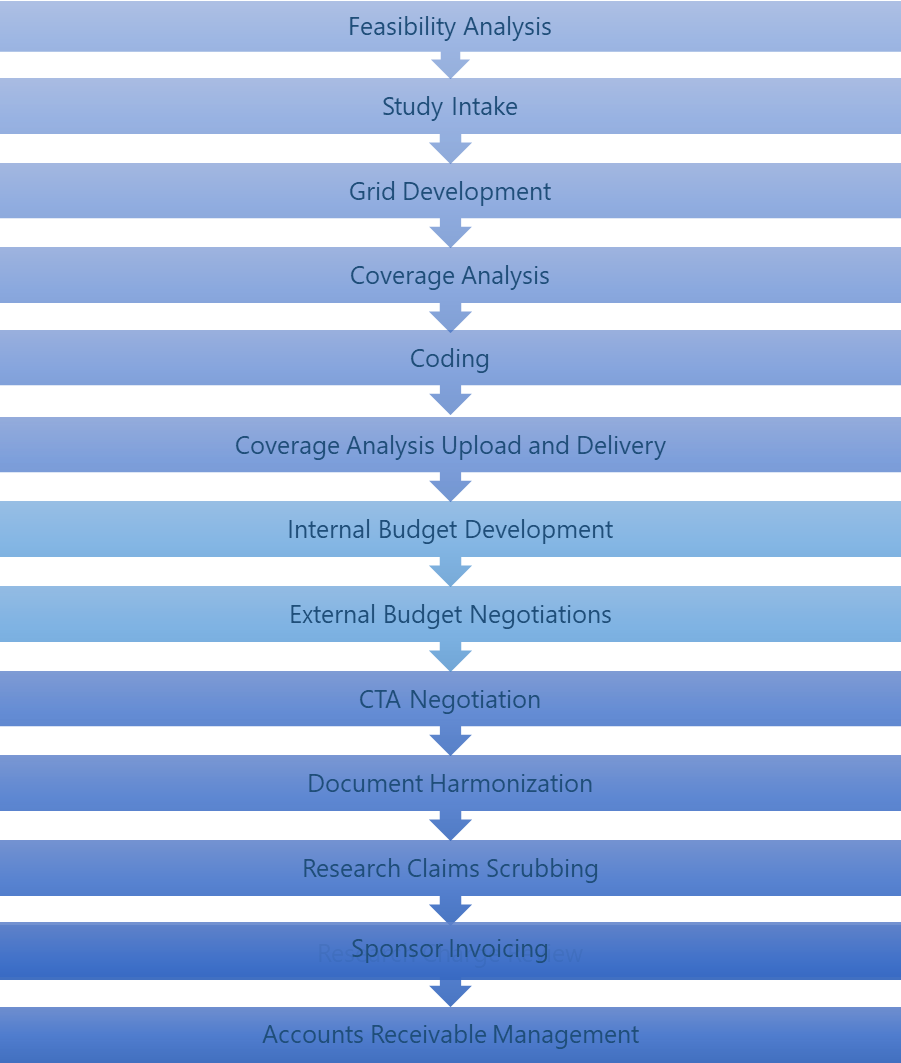
Pre-trial workstreams
Many of the ROC workstreams, from feasibility analysis through document harmonization, occur primarily before the trial begins. In order to ensure appropriate billing and the best contract terms from sponsors, it is essential that these workstreams are coordinated and completed prior to enrolling the first subject. There will also be times during the trial that some of these workstreams may need to be revisited and updated to ensure continued accurate billing. For example, when the protocol or the budget is amended, the coverage analysis (CA), coding, and document harmonization would need to be updated to reflect the new protocol items or new payment terms.
Feasibility analysis
While sponsors typically conduct a feasibility assessment for a proposed clinical study, research institutions do not always do so. As the clinical research landscape changes and financial margins decrease, research institutions should also consider whether the trial will be beneficial and aligned with its research portfolio and strategy.
A missing or insufficient feasibility assessment could lead to misalignment between the principal investigator (PI) and the institution, financial strain, stressed organizational resources and capabilities, and additional risk for the institution based on an insufficient study support structure.
Institutions with best practices have a documented and consistent feasibility analysis process that engages both the PI and research administration.
Compliance tips
-
If the institution does not currently have a study feasibility process, convene a working group to discuss and develop such a process.
-
Work toward a collaborative process that considers the expertise and priorities of the PI and the research priorities of the institution.
-
Consider:
-
Financial implications of the study;
-
Study alignment with the institution’s research strategy, portfolio and goals, and risk profile;
-
Sufficiency of needed resources, time, and capability of the PI, study teams, facilities, etc.;
-
Viability of patient recruitment and retention; and
-
History with and reputation of the sponsor and collaborators.
-
Study intake
Study intake is the process of determining whether the study will proceed through the research revenue cycle processes (i.e., whether the study has any protocol-required items or services that could generate a charge). Successful study intake also entails gathering and reviewing all of the study documents (e.g., protocol, draft informed consent form [ICF], draft clinical trial agreement [CTA]/budget offer, any Food and Drug Administration or Medicare administrative contractor documents, etc.) and storing them in a consistent way.
Inadequate study intake processes can result in disorganized communication and missing study documents and can put the institution at risk of not identifying a study that requires a billing CA, which could lead to improper billing.
Institutions with best practices have a centralized process for collecting and storing study documents and evaluating whether the study has any items or services that could generate a charge.
Compliance tips
-
Evaluate the current study intake process. Is it documented? Is there consistency across departments and locations?
-
A centralized process and centralized office for study intake will help ensure consistency across all clinical research.
-
Establish a process for collecting and storing study documents. Where do the documents come from? Who provides them and how? What system will be used to store the documents? Who will have access to the system?
-
Develop a process for determining whether any items or services could generate a charge, therefore requiring a CA. Decide who will make the determination, to whom the results will be reported, and how the study will enter the research revenue cycle process and proceed toward a CA.
Grid development
Grid development is the first step in the CA and budget negotiation processes. A grid captures all protocol-required items and services and their frequency and timing. The finalized grid provides a framework of all items and services that will occur during the trial—critical for the CA and budget negotiations. Most grids are built either in Excel or a clinical trial management system.
An inaccurate or inadequate grid could result in erroneous billing or missed sponsor invoice opportunities. If not all items and services are captured, the missed items/services could either be billed to the payer/patient when they should not have been, or the institution could miss out on the opportunity to negotiate payment for that item/service from the sponsor.
Institutions with best practices devote sufficient resources and develop standard processes in order to develop grids that accurately capture all study activities and their frequency.
Compliance tips
-
Start with the schedule of events, but do not stop there. The schedule of events should provide a snapshot of study activities, but most studies require items, services, or time points that are not captured in the schedule of events.
-
Additional items, services, or time points may be discussed in the footnotes of the schedule of events, in other areas of the protocol, in the ICF, or even in the draft funding documents.
-
Ensure that the institution’s grid development procedure considers all of the study documents, the PI’s understanding of the study events, and any peculiarities of the institution’s billing system.
-
Coverage analysis
A CA is a systematic approachto determine what, if any, charges in a study may be billable to a third-party payer according to National Coverage Determination (NCD) 310.1 and various regulations for device studies. The CA is built on the billing grid. The NCD 310.1 allows billing for certain items and services that meet the definition of “routine costs” that occur during a “qualifying clinical trial” when no other Medicare rule precludes billing.[4] Medicare standards are typically used when determining research study coverage, as Medicare has the most developed framework, most payers follow the lead of Medicare, and the consequences for billing errors are the most significant with Medicare.
An inadequate CA can lead to billing risks, including potential False Claims Act liability. The risks include:
-
Double billing, or billing for something the sponsor has agreed to pay for;
-
Billing for items or services promised free to the patient;
-
Billing for items or services that are performed for research purposes only;
-
Billing for certain items or services that are provided in a nonqualifying clinical trial (i.e., a trial that per NCD 310.1 does not qualify for coverage or a nonapproved device study); and
-
Billing for items or services that are not routinely covered outside a clinical trial.
Institutions with best practices develop consistent processes for determining what items and services in a clinical research study may be billable to the patient or third-party payer. The CA not only guides billing during the study, but also helps the institution negotiate a budget with the sponsor by requesting the sponsor pay for items/services for which the institution cannot bill.
Compliance tips
-
Develop a consistent and documented process for performing the CA. Consider developing tools like standard comments and positions on handling specific billing situations.
-
Ensure adequate training and education for those that complete the CA.
-
Institute a quality assurance process to peer review each CA before finalization.
-
Ensure that the CA process includes logic and supporting documentation for:
-
The qualifying clinical trial assessment that determines whether the trial qualifies for coverage;
-
Routine cost assessment to determine objective conventional care, what is needed for administration of the investigational item, and what items and services are provided to prevent, detect, or manage side effects of the investigational item;
-
Application of relevant Medicare rules; and
-
The unique variations between drug, device, and observational studies.
-
Coding
Once the CA is performed, the next step in the clinical research billing process is coding the items and services on the calendar grid for future billing purposes. The coding workstream involves having certified outpatient coders apply Current Procedural Terminology and Healthcare Common Procedure Coding System codes to the service lines on the calendar grid.
Compliance tips
-
The development of a basic coding library can be a useful tool for billing compliance purposes. A coding library lists the most common services seen in a calendar grid and the associated codes. This tool is then used during the coding process to efficiently code the calendar grid.
-
A customized library can provide further assistance to the institution’s claims scrubbers.
-
Including all codes related to a procedure also makes study budget development more accurate and efficient and reduces the risk of under-budgeting for items and services required to perform the study.
CA upload and delivery
Once the draft CA is complete, it is uploaded to the clinical trial management system, if used by the institution, and provided to the PI and study team for review and approval. Delivery should involve collaboration between the research revenue cycle and the PI/study team, as the PI can lend expertise and provide supporting documentation that the analysis team may not have been able to locate.
If the CA is finalized without collaboration with the study team, it is at risk of inaccuracy, as the PI is often the subject matter expert and can shed light on areas of the CA that may seem vague to the research revenue cycle.
Institutions with best practices have a collaborative CA delivery process that involves an open and ongoing dialogue between the CA and the study teams.
Compliance tips
-
Ensure adequate PI and study team education, including what a CA is and what it is used for, the risks of not billing correctly, the benefits of identifying what is not billable to aid in budget negotiations, and how the PI can contribute subject matter expertise to the CA.
-
Work to develop rapport between the study team and the coverage analyst. Ensure the study teams know how CAs help support the mission of the institution and that analysts are an available resource.
-
Allow the PI to weigh in on billing designations and reasonable and necessary determinations, as well as to provide additional support, if available.
-
Incorporate PI review and approval of the CA into the research revenue cycle.
-
Determine standard CA delivery (e.g., via email, secure site, clinical trial management system, or phone or in-person meeting) and consistent communication methods for responses to the CA.
-
Consider regular communications (e.g., newsletter) to PIs and study teams from the CA office to ensure updates are communicated and study teams understand processes and expectations.
Internal budget development
Internal budget development helps the institution estimate the total costs involved with participating in the study. The internal budget includes not only the cost of all the protocol-required items and services, as outlined in the CA, but also the various administrative costs incurred in the pursuit of the research. An internal clinical trial budget helps ensure the financial viability of the study. It also serves as the basis for sponsor negotiations, since it captures all study costs and can serve as a future audit tool. A consistent process for internal budget development can also help the institution negotiate acceptable budgets across other studies with the same sponsor and can ensure documentation and justification of costs in case of future audit.
The success of the internal budget is based on its attention to detail and its ability to capture all potential costs associated with the study. The primary pitfall of internal budget development is failure to account for all costs. Failing to or inaccurately capturing study-related costs will hamper the success of external budget development and sponsor negotiations and can result in decreased financial solvency of the study and the institution’s research program.
Institutions with best practices understand the importance of capturing all study-related costs and building a foundation for external budget negotiations. These institutions also use consistent processes and templates to establish a precedent for future study negotiations and help the organization anticipate research revenue, instead of accepting initial sponsor offers or sacrificing future negotiations to gain approval for the study at hand.
Compliance tips
-
Coordinate across departments that have insight into study costs. Ensure open communications between research administration and finance departments.
-
Ensure the internal budget includes not only the costs from the CA (protocol-required items and services), but also study-related administrative costs.
-
Develop an internal budget template that consistently anticipates research-related costs, such as subject treatment (protocol-required items and services), administrative efforts specific to the study, and necessary adjustments such as invoiceable options, departmental considerations, or expected inflation over the course of the study.
-
When developing the internal budget, review historical data such as past negotiated budgets and specific study-related costs to ensure this study is consistent with precedent and does not sell the institution short.
External budget negotiations
The goal of external budget negotiations is to get the maximum amount of financial support from the sponsor for the institution’s participation in the study. Favorable negotiations result in research programs that are positive contributors to the institution’s financial well-being.
Without solid external budget negotiations processes, the institution will likely not get maximum financial support from the sponsor, resulting in lower research revenues and an overall weaker research infrastructure.
Institutions with best practices have mature external budget negotiation processes that work toward maximizing the remuneration associated with the cost per patient, as well as the administrative, start-up, and overhead fees for which the sponsor should be expected to pay.
Compliance tips
-
Rather than focusing merely on covering basic costs associated with the study, mature negotiations view the contracting process as precedential, relying on previously negotiated budgets as the floor for future budgets.
-
Real-time intelligence is used so the highest payment received in the past is the lowest payment that will be accepted going forward.
-
Ensure that consistent justification for costs is documented.
-
Ensure that the budget planning and negotiations for multiyear studies include forecast increases for year-over-year adjustments.
CTA negotiation
CTAs are the contracts that bind parties in the context of a clinical trial. The parties involved include the institution(s) and the sponsor, and sometimes the PI.
An uninformed CTA negotiations process leads to unfavorable assignment of risks and disadvantageous delegation of costs and responsibilities.
Institutions with best practices understand their risk profile, consider obligations to their stakeholders, and examine all relevant issues, but they focus primarily on high-risk issues to balance the clinical trial risks and costs with the timeline constraints of contract negotiations.
Compliance tips
-
Be prepared to negotiate with the sponsor instead of just accepting the sponsor’s CTA with little or no negotiation.
-
Ensure negotiation of standard/key business terms such as indemnity, insurance, subject injury, etc.
-
Consider developing a template or checklist that outlines the institution’s key CTA positions.
-
Negotiate for the most favorable allocation of risk, cost, and responsibilities.
-
Ensure the CTA is aligned with both the CA and the budget.
-
Make sure the CTA language does not restrict the institution from billing for items or services that would have been billable according to NCD 310.1/the CA.
Document harmonization
Document harmonization is the process of harmonizing or syncing the final study documents, including the approved ICF and executed CTA or grant (and associated budget) with the draft CA. This prepares the CA to be used for billing and ensures that the CA accounts for any items or services promised free to the patient or paid by the sponsor or grant.
Institutions with no such harmonization expose themselves to risk, as the CA that will be used to guide billing may not incorporate the final financial terms described in the final study documents. Accurate interpretation of the final study documents’ billing language is also essential, as billing a service promised free or where sponsor/grant payment is promised can result in False Claims Act violations and subject the institution to penalties and sanctions.
Institutions with best practices have a workflow that ensures the draft CA is finalized by syncing it with the final study documents before it is used for billing.
Compliance tips
-
Develop a documented workflow that ensures the draft CA is updated with the final ICF and CTA terms prior to it being used for billing.
-
Ensure understanding by those responsible for billing that the CA cannot be applied until it has been synced with the final study documents. Develop a version-naming convention that indicates whether the CA version has been synced with the final study documents.
